In the Following Table Draw the Molecular Geometry
Draw the previous molecule with correct geometry 3. In some cases you may be.
Answered 12 In The Following Table Draw The Bartleby
In this experiment you will build models of molecules using a model kit.

. The second step is to calculate the BF3 hybridization and the third step is to give perfect notation for the. Key Points To Consider When drawing The BF3 Molecular Geometry A three-step approach for drawing the BF3 molecular can be used. Draw Lewis structures for the molecular formula given.
The first step is to sketch the molecular geometry of the KI molecule to calculate the lone pairs of the electron in the central iodine atom. Up to 24 cash back Draw Lewis structures AND predict the molecular geometry of the following compounds or polyatomic ions. What is the molecular geometry of chlorine trifluoride.
If there are resonance structures include their Lewis formulas. Chemistry questions and answers. The molecular geometry or shape for CH4 is the tetrahedral with bond angle H C H 1095.
Chlorine and fluorine come from. To chose the central atom as the one with the smallest number of valence electrons or if they all have the same number of valence electrons then choose the one in the least amount. A table of geometries using the VSEPR theory can facilitate drawing and understanding molecules.
We still have 12 pairplace 3 pair on each terminal Cl atom. The various molecular geometries for these types of molecules are shown in tables and described on the following pages. You will make models of the following molecules and draw a three-dimensional representation of their VSEPR molecular shapes into the table.
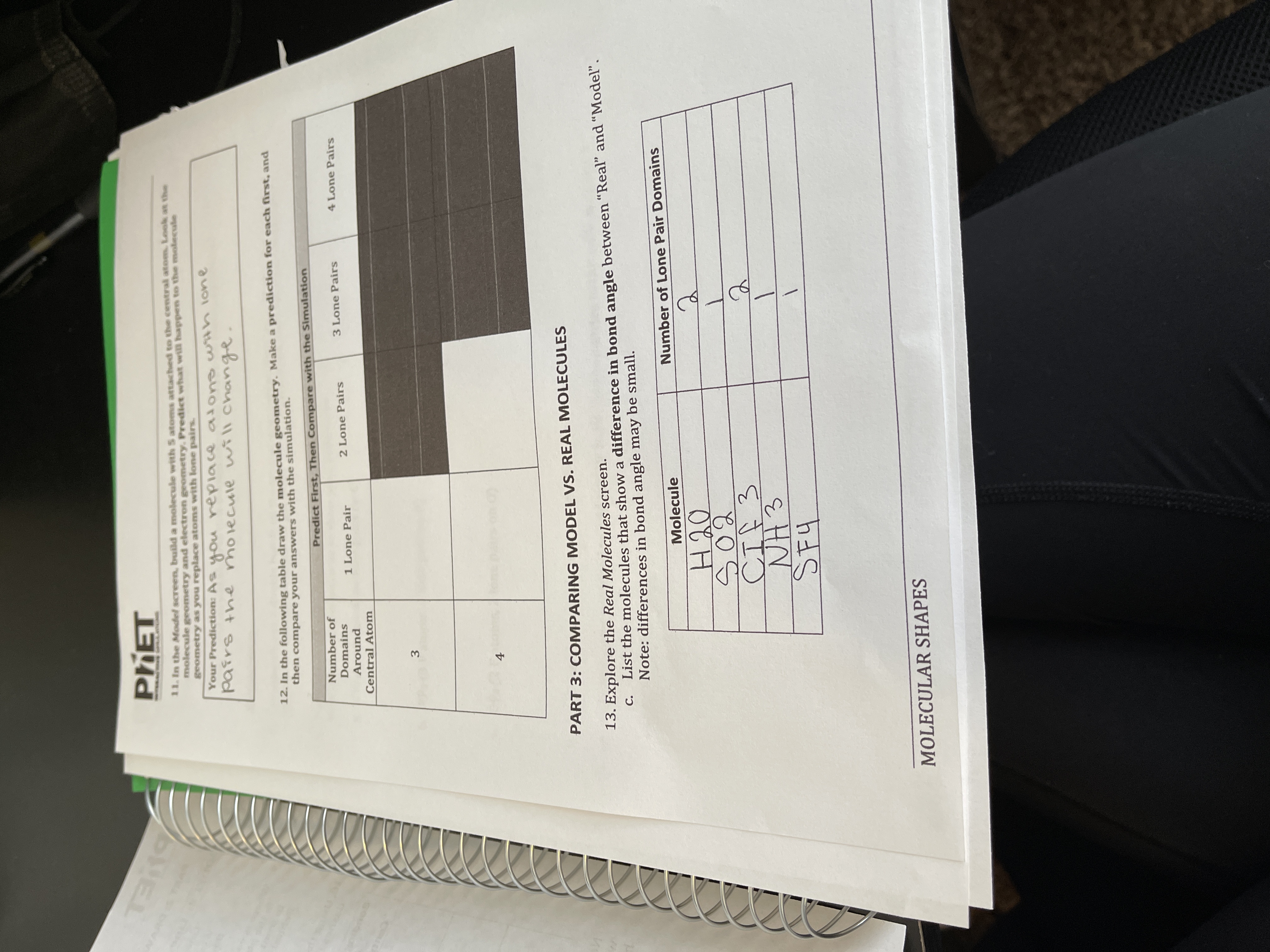
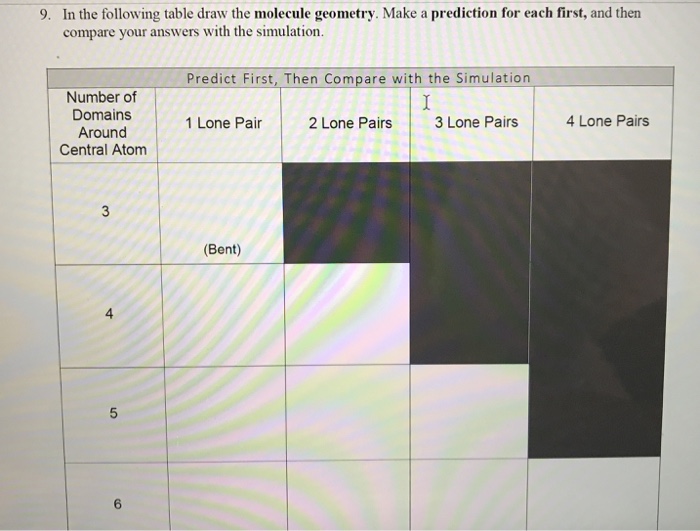
Predict First Then Compare with the Simulation Number of Domains Around Central Atom 1 Lone Pair 2 Lone Pairs 3 Lone Pairs 4 Lone Pairs. 10 Fill in the table below to determine the molecular geometry for the following molecules. 2 bonds 1 lone pair.
Chemistry 101 11-MOLECULAR GEOMETRY. The table of molecular geometries can be found in the first figure. Molecular geometries linear trigonal tetrahedral trigonal bipyramidal and octahedral are determined by the VSEPR theory.
As a group make a prediction for each first and then compare your answers with the simulation. Key Points To Consider When drawing The NH4 Molecular Geometry A three-step approach for drawing the NH4 molecular can be used. In the following molecule draw the correct Lewis structure.
Formula ABE formula Number of e-domains on central atom e - domains non-bonding domains on central atom Electron-Domain Geometry name Molecular Geometry name Bond angles on central atom CO 2 AB 2 2 2 0 Linear Linear 180 o. Electrons Required to give all atoms except hydrogen an octet and hydrogen a duet VE. The following table will help you understand how molecular geometry can be predicted using the VSPER model.
The first one has been completed as an example. Trifluoro chlorine or chlorine trifluorideClF3 has the composition of one chlorine and three fluorine atoms. 32 valence electrons or 16 pair C is in center with 4 Cls aroundthis uses 8 electrons or 4 pair.
SP 12 ER VE Hybridization. Draw Lewis structures for the molecular formula given. 2 Bonds 0 Lone Pairs 180 degree bond angle AB2 Type Ex.
Halogens and noble gases can expand their octet. Draw Lewis structures name the molecular. The Relationship Between the Number of.
In the following table draw the molecule geometry. Shared Pairs of electrons. Chemistry 1 Molecular Geometry Review.
The various molecular geometries for these types of molecules are shown in tables and described on the following pages. How to use the table to predict molecular geometry. Terms in this set 13 Linear.
26 rows Table Summarizing Molecular Geometries. 3 bonds 0 lone pairs 120 bond angle AB3 type Ex. Name_ Chem 121 Lab Molecular Geometry Exercise Part I.
In the following table draw the molecule geometry. Chemical formula Lewis structure Geometric sketch including bond angles CH4 OCl2 NCl3 CO2 CH2O. Molecular Geometries from each Electron Domain Geometry Electron Domain Geometry of Outer Molecular Atoms of Lone Pairs General Formula Geometry Name trigonal planar 3 0 AB 3 Trigonal planar trigonal planar 2 1 AB 2 E.
Of the central atom Molecular Geometry. Valence electrons available to all the atoms SP. As a group make a prediction for each first and then compare your answers with the simulation.
The second step is to calculate the KI hybridization and the third step is to give perfect notation for the KI molecular geometry. Bond Molecular Geometry around Central Atom hybridi- zation CFCHO angle FCC cco 2. To chose the central atom as the one with the smallest number of valence electrons or if they all have the same number.
CCl4 Tally the valence electrons C 1 4 4 Cl 4 7 28 Total. Also indicate the molecular geometry around the central atom the hybridization around the central atom and approximate bond angles. Molecular Geometries Where Central Atom Has Lone Pairs Continued Original Shape without Lone Pairs of Outer Atoms of Lone Pairs General Formula Molecular Geometry Name trigonal planar AB3 2 1 AB2E bent or angular 3 1 AB3E.
11- Draw the Lewis Structure and determine the electron geometry eg molecular geometry mg polarity and hybridization of XeF5. The first step is to sketch the molecular geometry of the BF3 molecule to calculate the lone pairs of the electron in the central boron atom. About the central atom PCl 3 Lewis structure ER VE SP hybridization molecular geometry 32 32.
For the following bonds identify which has the most covalent character. The second figure serves as a visual aid for the table. The second step is to calculate the NH4 hybridization and the third step is to give perfect notation.
Molecular Model Building Exercises Complete the following table by first drawing a Lewis structure and geometric sketch for each formula. View Chem121L Molecular Geometry Exercisepdf from CHEM 121L at North Dakota State University. Here in this post we described step by step to construct ClF3 molecular geometry.
The first step is to sketch the molecular geometry of the NH4 molecule to calculate the lone pairs of the electron in the central nitrogen atom. How to use the table to predict molecular geometry. Drawing and predicting the ClF3 molecular geometry is very easy by following the given method.
Build molecules following the table below and draw the molecule geometry with lines wedges and dashes. The electron geometry for CH4 is also tetrahedral as it central has 4 regions of electron density with no lone pair on it. A three-step approach for drawing the KI molecular can be used.
Solved 9 In The Following Table Draw The Molecule Geometry Chegg Com

Molecular Geometry Boundless Chemistry

Solved 11 In The Following Table Draw The Molecule Chegg Com
No comments for "In the Following Table Draw the Molecular Geometry"
Post a Comment